New research reveals psychedelic therapy recalibrates brain and immune system interactions, offering promising integrated treatments for anxiety, depression, and inflammatory disorders.
In recent years scientists have begun to understand that psychedelic therapy does more than expand consciousness – it also engages the immune system. Traditionally, psychedelics were studied solely for their effects on neural circuits and perception.
Now new research shows that compounds like psilocybin “reshape how the brain and immune system communicate”. This emerging view comes at a time when psychiatry is rethinking its narrow focus on neurotransmitters. In disorders from depression to PTSD, inflammation and immune signaling are often prominent, yet conventional treatments generally ignore this biological layer. Experts argue this gap calls for integrated models of mental health care – approaches that treat mind and body together, rather than separately.
Mental health’s immune dimension is now clear: chronic stress and illness reliably trigger inflammation that worsens mood and anxiety. Studies in people with depression or PTSD show elevated cytokines (like IL‑6, TNF-α and CRP) and overactive stress hormones. One recent human study found that healthy volunteers given psilocybin had an acute drop in the pro-inflammatory cytokine TNF-α and, days later, sustained reductions in IL-6 and CRP. In short, psychological stress and psychiatric symptoms often go hand-in-hand with an immune response, a reality missed by most antidepressants. Current treatments (SSRIs, SNRIs, psychotherapy, even ketamine) can work, but they “face challenges such as slow onset of action, wide individual variation, and inconsistent long-term efficacy”. This suggests that when brain–immune communication is disrupted, focusing on neurons alone may leave key biology “on the table”.
Psychedelics Rewire Brain–Immune Circuits
New findings indicate that psychedelic therapy may naturally address this mind–body gap. Rather than acting solely on neurons, classic psychedelics appear to reset entire neuroimmune circuits. In one pioneering study, researchers showed that chronic stress alters amygdala signaling and triggers immune cells, heightening fear and anxiety. Psychedelic compounds like psilocybin and MDMA reversed those changes, calming both neural fear responses and peripheral inflammation. As one summary put it: scientists have identified a pathway where “chronic stress disrupts amygdala signaling, triggering immune responses that increase fear and anxiety,” and – critically – “psychedelic compounds reversed this process”.
Key Facts from this research emphasize a shift in thinking. Psychedelics not only alter perception but also engage immune biology. In effect, they offer a dual action: modulating neural plasticity while dialing down harmful inflammation. This may explain why reported benefits of psychedelic therapy span mood disorders, addiction and even immune-related illnesses with psychiatric overlap. In the words of study authors, these compounds “help dial down inflammation and reset brain–immune interactions,” a finding that could “reshape how we think about treatment for inflammatory disorders and conditions like anxiety and depression”. More bluntly, Wheeler’s team argues that psychedelics recalibrate neuroimmune circuits, not just neurons, potentially offering a path to treatments that target both neural and immune dysfunction.
Psilocybin’s Dual Impact on Brain and Body
The serotonin-derived compound psilocybin vividly illustrates the dual action of psychedelic therapy. In controlled trials, a single dose of psilocybin in healthy volunteers caused an immediate drop in the pro-inflammatory cytokine TNF-α, and seven days later led to sustained reductions in IL-6 and CRP. These immune changes were linked to measurable mood improvements and lower stress-hormone (cortisol) responses. In parallel rodent studies, psilocybin (and MDMA) blocked the usual stress-induced immigration of immune cells (monocytes) into the brain’s protective membranes, preventing the inflammatory cascade that normally heightens fear. In stressed mice, this intervention translated to less freezing and anxiety in conditioned fear tests. In short, psilocybin delivered a one-two punch: rewiring the neural fear circuit while suppressing pro-inflammatory signals in the brain.

Amygdala and Astrocytes in Fear Signaling
Much of this effect traces back to the amygdala – the brain’s alarm center – and the glial support cells that modulate its activity. Under normal conditions, amygdala astrocytes (a type of glial cell) help put the brakes on fear by signaling through the EGFR receptor. Wheeler’s lab discovered that “astrocytes in the amygdala use a specific receptor called EGFR to limit stress-induced fear”. Under chronic stress, this astrocyte-based feedback loop breaks down, unleashing a cascade of neural excitation and inflammation that intensifies anxiety. Strikingly, psychedelics seem to restore this balance. In both animal models and human tissue, psychedelic treatment was shown to repair astrocyte signaling and interrupt the resulting neuroimmune loop. The result is reduced amygdala over-activity and fewer fear behaviors. As Wheeler summarizes, psychedelics can “reverse this entire process,” normalizing both neural and immune components of the fear response.
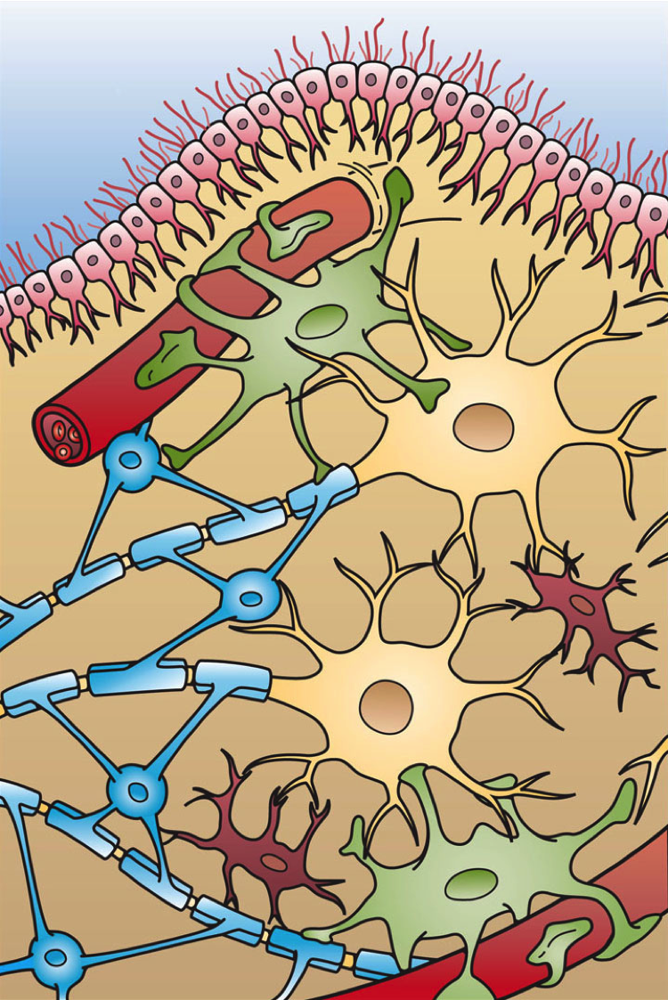
The cellular picture is complex but revealing. For example, lab studies have shown that psilocin (the active metabolite of psilocybin) directly dampens microglial activity. In cultured microglia, psilocin did not simply kill cells – it blocked their inflammatory functions. Specifically, microglia exposed to psilocin produced far less reactive oxygen and nitrogen species and showed reduced phagocytosis. Since overactive microglia contribute to brain inflammation, these findings suggest a mechanism by which psychedelic therapy actively suppresses neuroinflammation. In combination with its effects on neurons, this glial modulation may help explain why psilocybin and related compounds can have powerful, long-lasting effects on mood and fear.
Chronic Stress and the Neuroimmune Interface
Decades of work have shown that psychosocial stress engages the immune system, fueling inflammation that feeds back on the brain. In both animals and humans, prolonged stress raises peripheral cytokines and recruits immune cells to the brain’s protective meninges. Studies cited by neuroscientists note that stress-linked psychiatric patients often carry signatures of systemic inflammation. Wheeler’s Nature-backed experiments detailed this process: under chronic restraint stress, mice showed increased immune–brain cell “crosstalk” in the amygdala, driving fear behavior. Monocytes and other inflammatory cells invaded the meninges, amplifying amygdala neuron firing. In effect, stress rewired brain–immune communication to worsen anxiety.
Psychedelic therapy interrupts this cascade at multiple points. In Wheeler’s mouse models, treatment with psilocybin (or MDMA) prevented the usual monocyte build-up in the brain during stress. Immune signaling in the amygdala dropped, and fear behaviors diminished correspondingly. The treatment even dampened systemic stress markers like corticosterone. These findings were mirrored in human tissue: brain samples and patient datasets showed the same stress–immune gene changes seen in mice, implying clinical relevance. In short, psychedelic therapy reverses stress-induced inflammatory loops, recalibrating the neuroimmune interface back toward health.
Dr. Wheeler’s Interdisciplinary Breakthrough
Much of this science traces to the work of Dr. Michael A. Wheeler, a Harvard-trained neuroscientist turned immunologist. Wheeler’s team exemplifies the integrated approach now gaining traction: it brings together molecular neuroscience, immunology, and cutting-edge genomics. In Wheeler’s lab, experiments range from animal behavior and single-cell RNA sequencing to analysis of human brain tissue. For example, they used genomic screening and single-cell analysis to map exactly how stress disrupts amygdala circuits and calls in immune cells. The result is essentially a “wiring diagram” of brain–body communication under fear and stress.
The findings speak to Wheeler’s own journey. He began as a public defender interested in how environment shapes the mind, then deliberately learned immunology in graduate school. This cross-disciplinary path paid off: by “bringing together insights from neuroscience and immunology,” Wheeler uncovered previously hidden brain–immune channels that help explain why classic psychiatric treatments sometimes fail. In Wheeler’s view, psychedelic therapy works because it targets these neglected circuits on both sides. As he notes, psychedelic medicines may succeed where others stumble by addressing both neuronal and immunological pathways at once.
His lab’s published results underscore this paradigm shift. For instance, their recent Nature paper shows that psychedelics can normalize astrocyte signaling in the amygdala, block immune cell invasion of the meninges, and reduce anxiety. They report that interrupting one communication loop (astrocyte EGFR signaling) cascades into a broader neural and immune response – and that psychedelics reverse the entire chain reaction. Wheeler emphasizes that without cross-talk with immunology, brain research would have missed these dynamics entirely. It’s a powerful example of how team science and advanced tools (from genomics to cell imaging) are rewriting our understanding of brain disorders.
Technologies Mapping Brain–Immune Pathways
Behind the discoveries are state-of-the-art methods. Wheeler’s team employs genomic screens, single-cell transcriptomics, and even proteomic analyses to dissect the neuroimmune dialogue. For example, they isolate individual astrocytes and immune cells after stress or psychedelic treatment to see which genes switch on. They also use cell-type–specific manipulations: ablation of astrocyte EGFR or monocytes in mice let them prove each element’s role in fear signaling. These techniques allow researchers to chart, with unprecedented precision, how brain cells talk to immune cells during anxiety. The result is a “wiring diagram” of fear: neurons, astrocytes, microglia and invading immune cells linked by signaling pathways that either escalate or calm the threat response.
This integrative toolkit reflects a broader trend in neuroimmunology, where labs now routinely combine behavioral assays, brain imaging, cell biology and genetics. The goal is nothing less than a systems-level view: understanding depression or anxiety as emerging from a network of brain–body loops, rather than a single chemical imbalance. As Wheeler notes, it’s the convergence of different fields – from psychology to immunology – that will reveal why some patients improve with standard therapy and others do not. This knowledge will be essential to making psychedelic therapy precise and personalized.
Implications for Policy and Perception
The science is moving fast, and so is the world around it. Psychedelic therapy is gaining legitimacy both in medicine and public policy. In the U.S., two states have already legalized psychedelic-assisted treatments (for example, Oregon approved psilocybin therapy clinics) and Congress has expanded research on psychedelics for veterans. High-profile clinical trials (such as MDMA for PTSD and psilocybin for depression) are under way, although regulatory approval remains pending. Meanwhile, attitudes are shifting: a 2024 survey of 879 U.S. healthcare professionals found strong belief in the promise of psilocybin and MDMA therapy, with most respondents supporting legal access. Even among those new to the field, many say conventional treatments often miss the “whole biology” of mood disorders – an insight now echoed by researchers.
Nonetheless, stigma and legal hurdles persist. Psychedelics remain federally Schedule I drugs, and some lawmakers worry about safety or abuse. In California, for example, bills to legalize therapeutic psychedelics have stalled amid concerns over implementation and public health. Advocates are hopeful that burgeoning evidence will change minds: if psychedelic therapy can be shown to treat inflammation-related illness and mental health together, its appeal could extend to patients and providers who remain skeptical.
In practice, these findings suggest a new path for integration. Clinics offering psychedelic therapy may soon incorporate biomarkers of inflammation or genetics into patient screening and treatment plans. Insurance companies and regulators will have to adapt to therapies that straddle psychiatry and immunology. Ultimately, as Wheeler and others emphasize, the psychedelic renaissance might herald a deeper revolution: reconceiving mental health care as inherently interdisciplinary, grounded in both brain chemistry and immune biology.
Conclusion: Toward Integrated Care
Psychedelic therapy is more than a novel drug modality: it is forcing medicine to rethink the boundaries between mind, brain and body. We have seen how classic psychedelics act on neurons and on immune cells, undoing the chronic stress-driven inflammation that often underlies anxiety and depression. In doing so, they exemplify the kind of personalized, integrated care that modern psychiatry increasingly needs. In the coming years, research will expand these insights – for example, exploring which patients have high inflammatory burdens and might benefit most from psychedelics, or testing combinations of psychotropics and immunomodulators. Policymakers and practitioners will watch closely: as one expert put it, targeting neuroimmune circuits may ultimately prove more effective than focusing on neurons alone.
What is clear is that the story of psychedelic therapy is still unfolding. Each new study peels back another layer of the brain–immune dialogue. By continuing to chart these connections and treating mental health as a systemic issue, we may at last unlock treatments for the many sufferers that conventional psychiatry has left behind. The journey ahead is long, but the map is finally taking shape.

***
Trap Culture is the ultimate destination for cannabis enthusiasts who want to experience the best of Arizona’s cannabis culture. Whether you are looking for the hottest cannabis-friendly events, the latest news on cannabis legalization, trends in the industry and exclusive, limited-edition products from the top brands in the market, Trap Culture has you covered. Visit our website to learn more about our events, our blog, and our store. Follow us on social media to stay updated on the latest news and promotions. Join the Trap Culture family and experience the most immersive and engaging social cannabis events in Arizona.
Follow us on social media




